-
{{ cart_product.name }}
{{ Number(cart_product.price*cart_product.quantity).toLocaleString() }} UAH ×
Ion-Pair Reagents for HPLC
Ion-exchange chromatography systems have previously been utilized in HPLC analysis of ionic samples. Recently, reversed phase partition chromatography using ion-pair reagents has been developed and utilized. The ionic samples form an ion-pair with ion-pair reagents in the mobile phase to become electrically neutral. The increase in hydrophobic character of the ion-pair results in a greater affinity for the reverse stationary phase and leads to sample resolution.
UV and fluorescence detectors are widely used. Therefore ion-pair reagents must lack UV absorption and fluorescence themselves to obtain highly sensitive detection of samples. The UV absorption of sodium alkanesulfonates and quaternary ammonium salts is minimal so that these reagents can be used for reliable HPLC analysis. On the other hand, when a sample lacks sufficient UV absorption or fluorescence, the use of sodium 9,10-dimethoxyanthracene-2-sulfonate allows for high-sensitivity detection as a fluorimetric ion-pair reagent.
Recently, use of LC-MS in which mass spectrometry is incorporated in HPLC as a detector has become widespread. Sodium alkanesulfonates, a general ion-pair reagents, being non-volatile crystals pose a problem in that they contaminate the interface. The IPC-PFFA series is made of highly volatile ionpair reagents allowing for continuous LC-MS analysis without contaminating the interfaces.
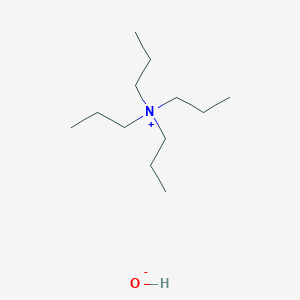
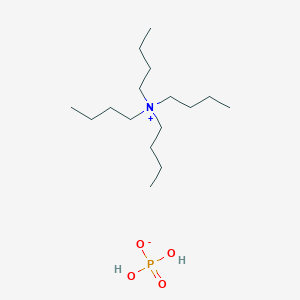
HPLC Ion-Pair Reagents for Acidic Samples
- Analysis is performed with pH adjusted to 7.5 with the addition of quaternary ammonium salts to the mobile phase.
- Acidic samples form an electrically neutral ion-pair with the quaternary ammonium salt and are retained in the reverse phase systems.
- The ion-pair reagents for acidic samples for LC-MS are supplied as 0.5 M aqueous solutions and were adjusted to pH 7.5. The solution can be used as a neutral mobile phase after dilution with the LC solvents (acetonitrile/water or methanol/water) to 5 mM. Since the acidic substances are ionized under the neutral conditions, they are facilitated to form an ion-pair.
[Examples]
1. When 0.5mol/L Tetrabutylammonium Phosphate (IPC-TBA-P) [I0367] is used:
The reagent (10 mL) is diluted to 1 L with an aqueous solvent such as methanol - water. (pH adjustment is not required because the reagent is already buffered.)
2. When Tetrabutylammonium Hydroxide (IPC-TBA-OH) [I0364] is used:
1) The reagent (12.5 mL) is diluted to 1 L with an aqueous solvent such as methanol - water.
2) The pH is adjusted to 7.5 by the addition of an aqueous phosphoric acid (10%).
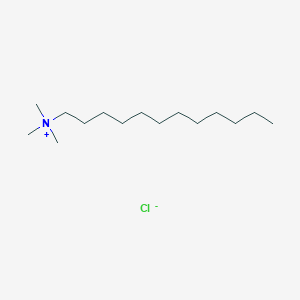
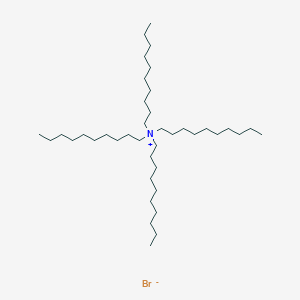
HPLC Ion-Pair Reagents for Basic Samples
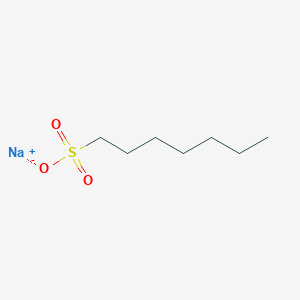
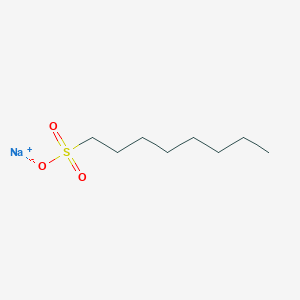
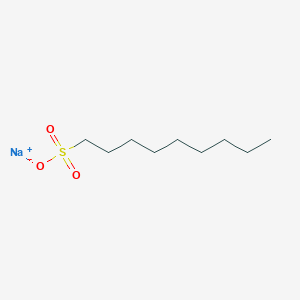
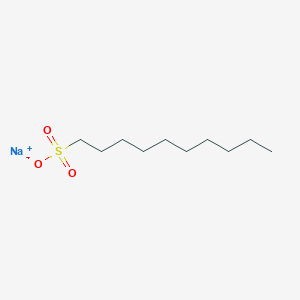
- Analysis is performed by the addition of sodium alkanesulfonate to the mobile phase.
- The basic samples form an electrically neutral ion-pair with sodium alkanesulfonate and are retained in the reverse phase system.
- In the case of sodium alkanesulfonate, the greater the number of carbons in the alkyl group, the greater the partition ratio.
- The solubility of the products such as sodium 1-decanesulfonate (IPC-ALKS-10) [I0348] may decrease depending upon the composition of the mobile phase solvents; especially after the addition of the buffer for pH adjustment. Resulted turbidity of the mobile phase and crystal formation may interfere with the analysis. To avoid the trouble, modification of the solvent system composition should be considered.
- The ion-pair reagents for basic samples in LC-MS analysis are supplied as 0.5 M aqueous solutions. The solution can be used as an acidic mobile phase after dilution with the LC solvents (acetonitrile/water or methanol/water) to 5 mM. Since the basic substances are ionized under the acidic conditions, they are facilitated to form an ion-pair.
- We supply the high-quality products (IPC-PFFA-6 HG [A5722], IPC-PFFA-7 HG [A5721], IPC-PFFA-8 HG [A5720]) of IPC-PFFA-6, IPCPFFA-7 and IPC-PFFA-8 for high-sensitive detections.
[Examples]
1) Sodium 1-Heptanesulfonate (IPC-ALKS-7) [I0345] 1.011 g (0.005 mol) is weighed out.
2) The reagent is dissolved in 1 L of an aqueous solvent such as methanol - water.
3) The pH is adjusted to 3.5 by the addition of aqueous phosphoric acid (50%).
Fluorimetric Ion-Pair Reagent
When a sample lacks sufficient UV absorption or fluorescence, the use of IPA-DAS [A5701] allows for high-sensitivity detection as a fluorimetric ion-pair reagent.